Abstract
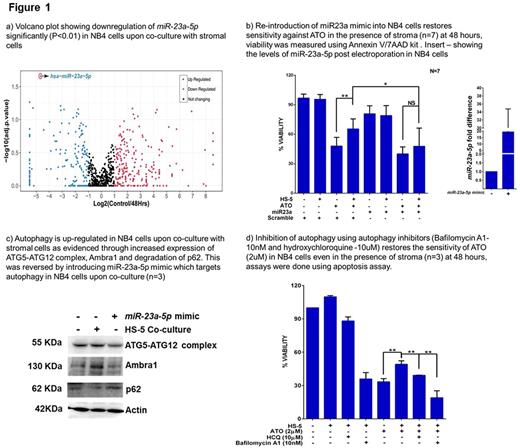
Role of stromal micro-environment in drug resistance has been extensively reported for several cancers. We have demonstrated earlier that there is significant microenvironment mediated drug resistance (EMDR) to arsenic trioxide (ATO) in acute promyelocytic leukemia (APL) and that this was predominantly driven by up-regulation of the NF-kB pathway in the malignant cell (Ganesan S et al. Leukemia 2016). In our current study we have probed the molecular mechanism of EM-DR in further detail. Towards this we evaluated the potential role played by miRNA in EMDR to ATO in APL. Using NGS based small RNA sequencing we identified hsa-miR-23a-5p to be significantly downregulated (adjusted P value <0.01; figure 1a) in NB4 cells upon co-culture with HS-5 stromal cells. We noted that this miRNA is negatively regulated by NF-kB pathway and inversely correlated with NF-kB status in relapsed APL patients (n=8; matched samples). These results were consistent with our earlier reported observations that NF-kB pathway is dysregulated and enhances drug resistance to ATO. Further, we observed that low expression of miR-23a-5p observed in the relapsed patient's samples showed a trend towards an increased association with subsequent second relapse.
We also observed miR-23a-5p mimics were able to restore the sensitivity of NB4 cells to ATO in the presence of stromal cells (figure 1b), while NB4/GFP-MAD cells (NF-kB inhibited cell line showing high expression of miR-23a-5p ) were not protected from ATO on stromal co-culture. Looking for the targets of miR-23a-5p , TargetScan analysis data demonstrated a clustering of targets involving toll like receptor genes (TLR) and autophagy pathway genes (TLRs - TLR2, TLR3, TLR4 and TLR5 and autophagy pathway genes - AMBRA1, ATG13, ATG9 and ATG2 ). Previously reported data clearly demonstrated the ability of TLR genes to activate autophagy (Delgado MA. et al. EMBO J 2008). Based on this data we evaluated the role of autophagy in EM-DR. We noted that there was an upregulation of autophagy pathway in leukemic cells upon co-culture with stroma (where miR-23a-5p was downregulated). Re-introducing miR-23a-5p in the leukemic cells targeted autophagy pathway genes which was validated through western blot (figure 1c). We also observed that inhibiting autophagy by chemical inhibitors (bafilomycin A1 and hydroxychloroquine) along with ATO was able to overcome EM-DR to ATO (figure 1d). The in-vivo efficacy of combining ATO along with hydroxychloroquine was further validated in an APL mouse model where we were able to demonstrate a significant decrease in tumor burden on day20 when compared to controls and significantly prolonged the survival of APL mice treated with the combination in comparison to either drug alone.
Similar results were obtained with AML cells, where the stromal cells conferred a protective effect over U937 cells against cytarabine (AraC) and daunorubicin (Dnr). The downregulation of miR-23a-5p was also observed in U937 cells upon co-culture. We also observed that re-introducing miR-23a-5p in these cells restored sensitivity to AraC and Dnr. Our results thus illustrates a complex molecular cross-talk between stromal cells and leukemic cells in protecting against the cytotoxic effects of various chemotherapeutic agents in myeloid leukemia. This data along with that reported earlier by us illustrates multiple levels of regulation of the NF-kB pathway and resistance to chemotherapeutic drugs by stromal cell co-culture. Our work thus suggests that miR-23a-5p mimics or autophagy-inhibitors could potentially be effective adjuvants along with chemotherapy/ATO in the treatment of myeloid malignancies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal